進口代理─美國蒙大拿州─「Solo-Dex」急性疼痛管理專利導管
- 發布日期:
- 瀏覽人次:
400
- 國名:美國蒙大拿州
- 公司名稱:Solo-Dex
- 公司所在:Montana
- 產品/技術:急性疼痛管理專利導管
- 官網:http://www.solo-dex.com
- 產業別:醫療器材
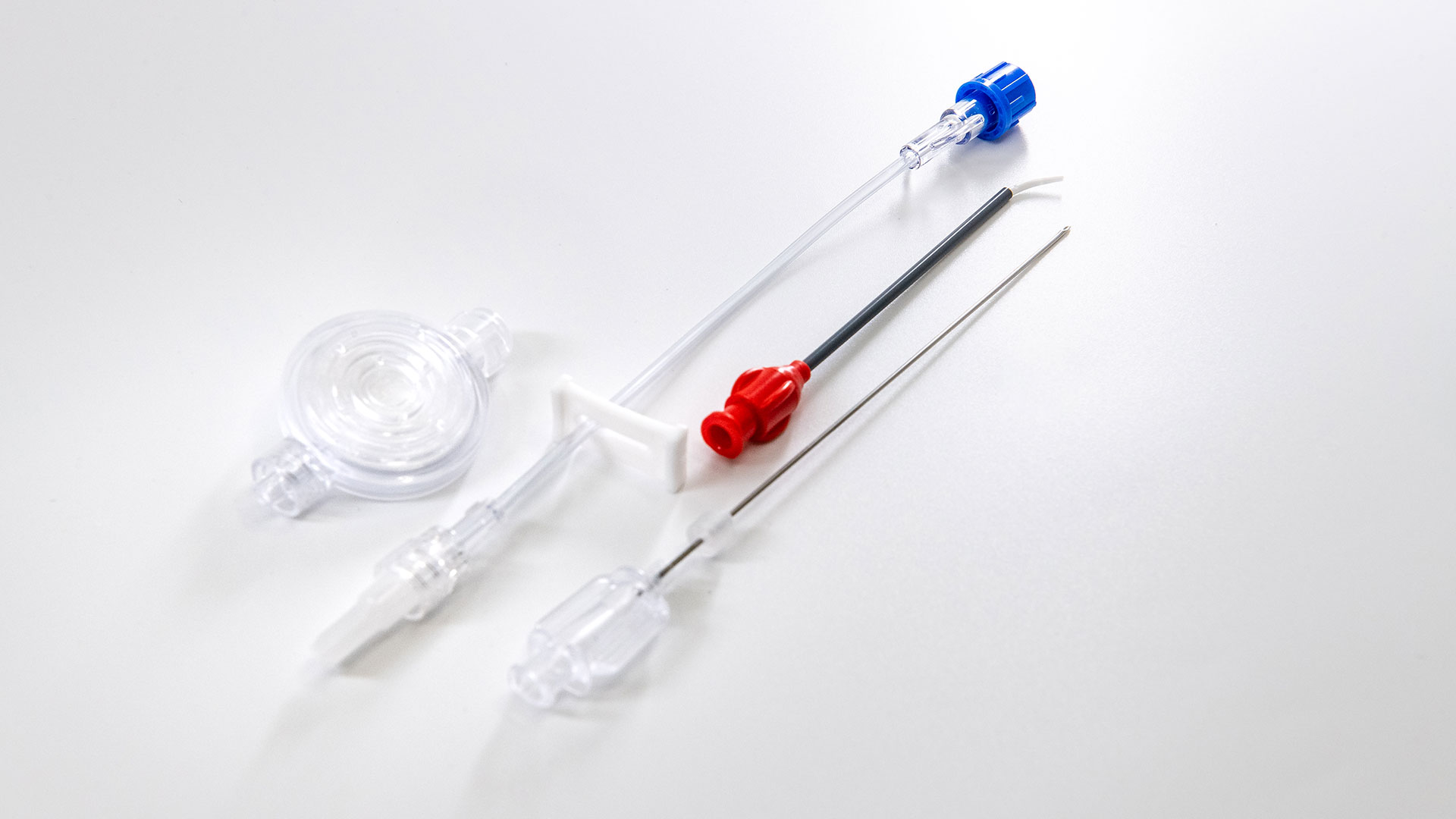
Solo-Dex 是一家先進醫療產品製造公司,明星商品的 Solo-Dex Fascile 專利導管,提供對持續周邊神經阻斷(cPNB)的開創性方法,協助術前、術中及術後的疼痛管理,可精確並持續地緩解急性疼痛。Solo-Dex 作為價值 40 億美元的急性疼痛管理市場中的創新者,擁有 17 項專利保護,獲得 FDA 的批准/ CE 標記。
Solo-Dex Fascile 專利導管在術後急性疼痛管理所能帶來的好處如下:
• 減少了對鴉片類藥物的需求
• 減少麻醉風險來提高病人的安全並可更快的康復和出院
• 為醫療設施創造成本效益並減少手術團隊的感染風險
Solo-Dex stands at the forefront of anesthesiology innovations, manufacturing, and distributing advanced medical products. Our crown jewel, the Solo-Dex Fascile catheter range, offers a groundbreaking approach to continuous Peripheral Nerve Block (cPNB). This device is specifically designed to manage acute pain before, during, and after surgery, thereby decreasing the need for opioids.As a key innovator in the $4 billion acute pain management market, Solo-Dex thrives on the global stage. Our innovative products, protected by 17 patents are FDA-approved and CE-marked.
Our goal is to redefine the paradigm of acute post-surgical pain management through the following pathways:
• Bolstering patient safety by reducing anesthesia-associated risks
• Facilitating quicker recovery and hospital discharge
• Promoting effective home recovery with a lowered dependency on opioids
• Creating cost-efficiency for healthcare facilities
• Enhancing patient satisfaction
• Diminishing infection risks for the surgical teams
聯繫窗口
聯絡人:美國蒙大拿州亞太商務辦事處 王美美代表
電郵:MeiMei.Wang@mt.gov
電話:02-27231732