進口代理─美國蒙大拿州─「DermaXon」皮膚與神經疾病治療藥品
- 發布日期:
- 瀏覽人次:
304
- 國名:美國
- 公司名稱:DermaXon
- 公司所在:Montana
- 產品/技術:皮膚致命疾病新療法
- 官網:http://www.dermaxon.com
- 產業別:西藥
DermaXon 是一家藥物探索和開發公司,提供一流的候選藥物。DermaXon 著重於大腦與皮膚間的連結,以探索並開發新療法去治療皮膚的致命疾病及症狀。
DermaXon 的核心技術在於多藥理學的小分子設計開發,以多靶點調控或抑制內源性分子降解的方式為基礎。這種多藥理學的方法旨在了解和優化現有小分子的未知脫靶活動。
本公司的主要技術包括:
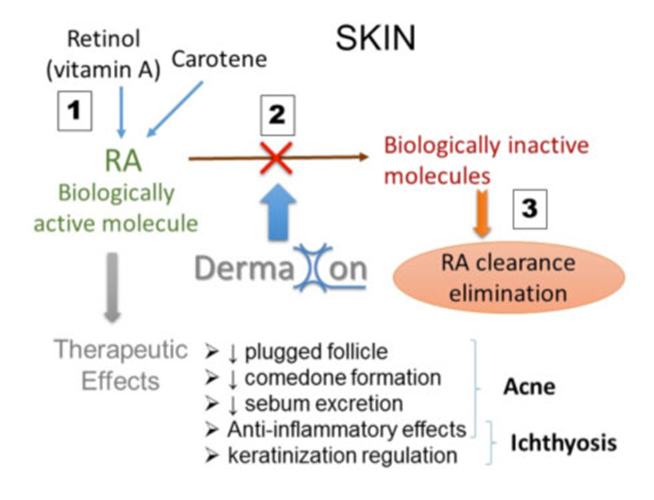
- DX416:一種治療皮膚疼痛和瘙癢的新型療法。作為孤兒藥所獲的稅收抵免及費用減免,有效降低DX416 的成本。該療法可用於市場上的大量皮膚病,包括:皮膚疼痛、皮膚搔癢及皮膚罕見病等。
- DX308:DX308 是一種用於治療罕見皮膚病--先天性魚鱗癬的新型療法。作為孤兒藥所獲的稅收抵免及費用減免,有效降低 DX308 的成本。該療法可用於尚未獲得 FDA 批准藥物的罕見疾病市場。
- DX814:透過納摩爾抑製劑將一種可藥物化的激酶 RIOK3 做為標靶,DX814 可應用於治療自身免疫性或超免疫性疾病。
 |
 |
DermaXon is a Montana-based drug discovery and development company founded in 2013 providing first in-class drug candidates. DermaXon targets the brain-skin connection to discover and develop novel therapeutic strategies to treat life-threatening diseases and disorders such as pain, pruritus and genetic disorders of skin keratinization.
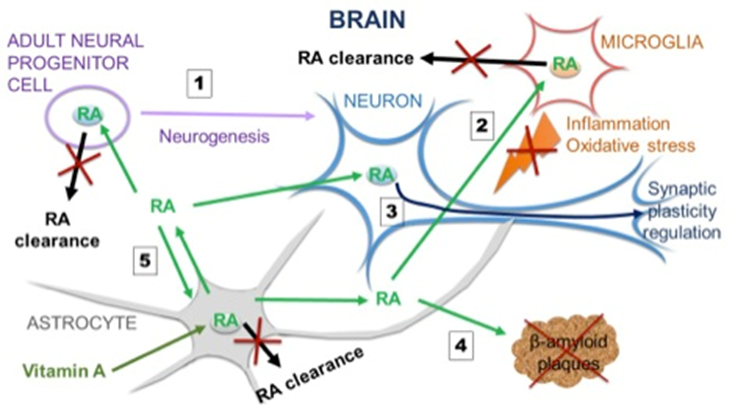
The company core technology is based on the design and development of small molecules modulating multiple targets or inhibiting the degradation of endogenous molecules exhibiting polypharmacology. This polypharmacological approaches aim to understand and optimize the unknown off-targets activities for existing small molecules such as cannabinoids or retinoic acid.
Platform Technology:
• DX416: a novel treatment for skin pain and pruritus. DX416 efficacy and tolerability: In vivo administration of DX416 has shown high efficacy at reducing pain and pruritus in vivo and ex vivo. Cost: No expensive manufacturing cost, tax credit and fees waivers will be obtained with the orphan drug status. Market: Skin pain and pruritus are associated with a large number of skin diseases including rare diseases
• DX308: DermaXon is developing DX308, a novel patent protected treatment of the rare skin disease, congenital ichthyosis. In vivo administration of DX308 has shown high efficacy without inducing erythema or toxicity. Cost: No expensive manufacturing cost, tax credit and fees waivers will be obtained with the orphan drug status. Market: Targets a rare disease market without FDA approved treatments.
• DX814: DermaXon has identified a nanomolar inhibitor (DX814) targeting an understudied druggable kinase RIOK3 that regulates interferon synthesis for the treatment of autoimmune or hyperimmune response disorders.
聯繫窗口
聯絡人:美國蒙大拿州亞太商務辦事處 王美美代表
電郵:MeiMei.Wang@mt.gov
電話:02-27231732